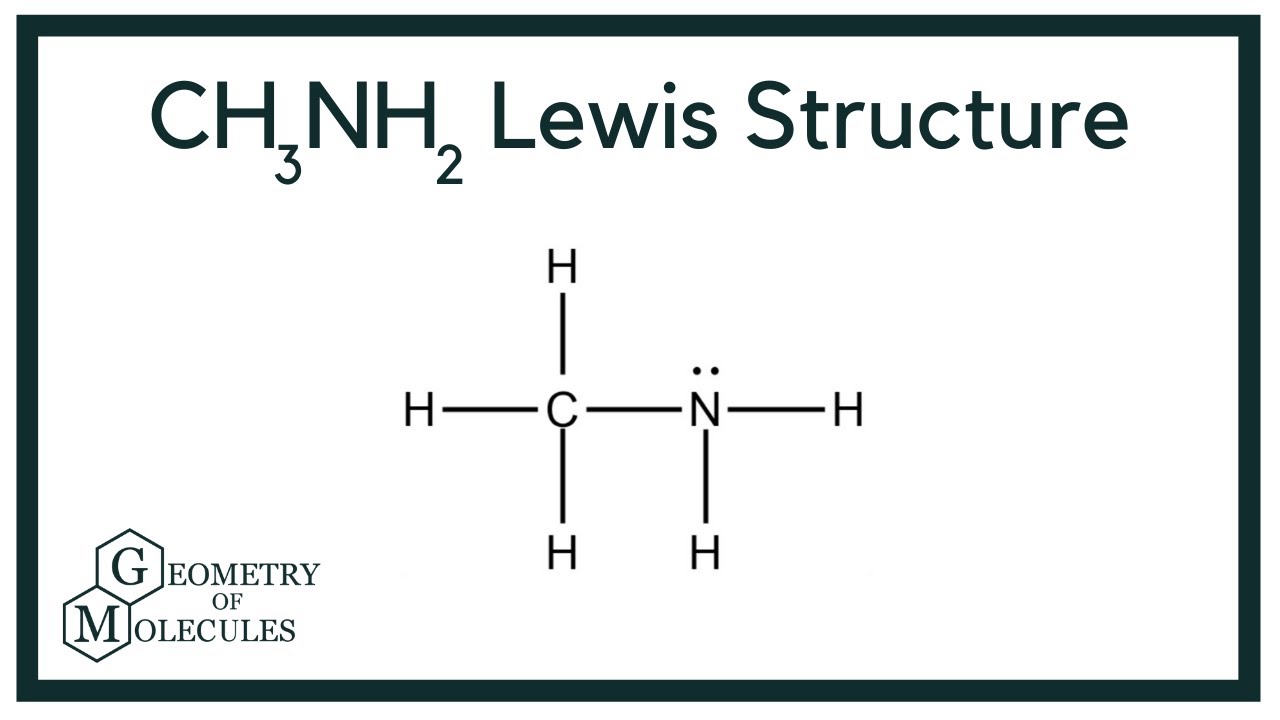
The CH3NH2 Lewis structure is a fundamental concept in chemistry that represents the arrangement of atoms in the methylamine molecule. Understanding this structure is crucial for students, chemists, and anyone interested in the molecular composition of compounds. In this article, we will delve deep into the details of the CH3NH2 Lewis structure, its significance, and the various aspects that define its molecular geometry and bonding.
The CH3NH2 molecule, also known as methylamine, is an organic compound belonging to the amine family. It consists of a methyl group (CH3) attached to an amino group (NH2). The Lewis structure of CH3NH2 provides insights into the electron distribution and bonding characteristics within the molecule. By exploring this structure, we can better understand its chemical properties, reactivity, and applications.
This article will cover the essential aspects of the CH3NH2 Lewis structure, including its formation, molecular geometry, and comparison with similar compounds. We will also discuss the significance of this structure in real-world applications, making it a valuable resource for anyone looking to deepen their knowledge of organic chemistry.
Table of Contents
- What is a Lewis Structure?
- Introduction to CH3NH2
- Drawing the Lewis Structure of CH3NH2
- Molecular Geometry of CH3NH2
- Polarity and Intermolecular Forces
- Comparison with Similar Molecules
- Applications of CH3NH2
- Conclusion
What is a Lewis Structure?
A Lewis structure is a diagram that shows the bonding between atoms of a molecule and the lone pairs of electrons that may exist. Named after the American chemist Gilbert N. Lewis, this representation provides a visual understanding of how atoms are connected and how they share electrons. In a Lewis structure, dots are used to represent valence electrons, while lines represent the bonds between atoms.
Introduction to CH3NH2
CH3NH2, or methylamine, is a simple aliphatic amine derived from ammonia. It plays a crucial role in organic chemistry and has various applications in the chemical industry. Methylamine is a colorless gas with a fishy odor, and it is soluble in water, alcohol, and ether. Its chemical formula indicates that it contains one carbon atom, one nitrogen atom, and five hydrogen atoms.
Data and Personal Information
| Property | Details |
|---|---|
| Chemical Formula | CH3NH2 |
| Molecular Weight | 31.06 g/mol |
| Boiling Point | 6.7 °C |
| Melting Point | -93.5 °C |
| Density | 0.785 g/cm³ |
Drawing the Lewis Structure of CH3NH2
To draw the Lewis structure of CH3NH2, follow these steps:
- Count the total number of valence electrons available in the molecule. Carbon has 4, nitrogen has 5, and each hydrogen has 1. Thus, the total is 4 (C) + 5 (N) + 2 × 1 (H) = 11 valence electrons.
- Identify the central atom. In CH3NH2, carbon is typically the central atom.
- Connect the atoms with single bonds. Connect the carbon atom to three hydrogen atoms and to the nitrogen atom.
- Distribute the remaining electrons to satisfy the octet rule. In this case, place the remaining electrons around the nitrogen atom to complete its valence shell.
- Check the structure to ensure that all atoms have the correct number of electrons. Carbon will have 4 bonds, nitrogen will have 3 bonds (with one lone pair), and each hydrogen will have 1 bond.
Molecular Geometry of CH3NH2
The molecular geometry of CH3NH2 can be described using the VSEPR (Valence Shell Electron Pair Repulsion) theory. The presence of lone pairs and bonded atoms affects the shape of the molecule. For CH3NH2:
- Central Atom: Carbon
- Bonding Pairs: 4 (3 from hydrogen and 1 from nitrogen)
- Lone Pairs: 1 on nitrogen
This results in a trigonal pyramidal shape due to the repulsion between the lone pair and the bonding pairs. The bond angles are approximately 107 degrees.
Polarity and Intermolecular Forces
CH3NH2 is a polar molecule due to the electronegativity difference between carbon, nitrogen, and hydrogen. The nitrogen atom pulls the electron density away from the hydrogen atoms. This polarity leads to hydrogen bonding, which is significant in determining the physical properties of methylamine, such as its boiling point and solubility.
Comparison with Similar Molecules
When comparing CH3NH2 with similar molecules, we can look at compounds like CH4 (methane) and NH3 (ammonia). Here are some key differences:
- CH4 (Methane): Non-polar, tetrahedral geometry, no hydrogen bonding.
- NH3 (Ammonia): Polar, trigonal pyramidal geometry, exhibits strong hydrogen bonding.
Methylamine shares characteristics with both, but its unique structure and bonding result in distinct chemical behavior.
Applications of CH3NH2
Methylamine has various applications across different industries:
- Used in the synthesis of pharmaceuticals and agrochemicals.
- Serves as a building block for the production of dyes and surfactants.
- Acts as a solvent in chemical reactions.
Its significance in organic chemistry cannot be overstated, as it plays a role in the development of various products and materials.
Conclusion
In conclusion, the CH3NH2 Lewis structure is pivotal in understanding the molecular composition of methylamine. By grasping the concepts discussed in this article, readers can appreciate the intricacies of molecular geometry, polarity, and the applications of this compound. As we continue to explore the world of chemistry, understanding structures like CH3NH2 will enhance our knowledge and application of chemical principles.
If you found this article helpful, please leave a comment or share it with others who may also benefit from learning about the CH3NH2 Lewis structure. Additionally, explore our other articles for a deeper dive into organic chemistry and related topics!
Thank you for visiting our site! We hope to see you again soon for more insightful content.
Nihilism Meaning: Understanding The Depth Of A Philosophical Concept
Exploring Osaka Pink Salon: A Unique Beauty Experience In Japan
Is Leah Williamson Married? A Comprehensive Look Into Her Personal Life